Ingenious Chemistry
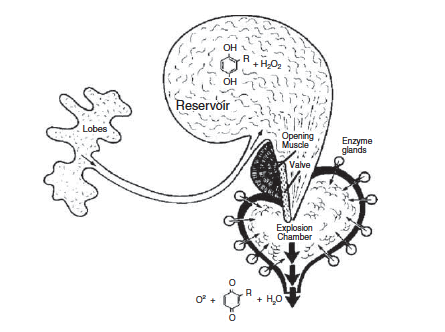 Nobody does chemistry like Metrius contractus does chemistry.
Not even other bombardier beetles have the same chemical make up
in their sprays (Eisner et al. 1977). The initial chemical
reactions of almost all bombardier beetles are the same, but in
the final result, Metrius contractus shows several key
differences (Eisner et al. 2000). First, it is essential to understand the basic chemistry present
across bombardier beetles in general. In the
reservoir chamber,
the chemicals that are present are
hydroquinone and
hydrogen
peroxide in solutions at concentrations of 25% and 10% by mass
respectively (Beheshti and McIntosh 2007). Quinone is formed in
aqueous solution initially and then stored in more of a lipoidal
form as a
hydrocarbon; therefore, both
aqueous and lipoidal phases are found in the reservoirs (Eisner
et al. 2000). As seen
in the discussion of
form and function,
enzymes
are also necessary for these reactants to do anything. Enzymes are present in
the
reaction chamber.
More specifically most of the enzymes
are
catalase and
peroxidase (Beheshti and McIntosh 2007).
Nobody does chemistry like Metrius contractus does chemistry.
Not even other bombardier beetles have the same chemical make up
in their sprays (Eisner et al. 1977). The initial chemical
reactions of almost all bombardier beetles are the same, but in
the final result, Metrius contractus shows several key
differences (Eisner et al. 2000). First, it is essential to understand the basic chemistry present
across bombardier beetles in general. In the
reservoir chamber,
the chemicals that are present are
hydroquinone and
hydrogen
peroxide in solutions at concentrations of 25% and 10% by mass
respectively (Beheshti and McIntosh 2007). Quinone is formed in
aqueous solution initially and then stored in more of a lipoidal
form as a
hydrocarbon; therefore, both
aqueous and lipoidal phases are found in the reservoirs (Eisner
et al. 2000). As seen
in the discussion of
form and function,
enzymes
are also necessary for these reactants to do anything. Enzymes are present in
the
reaction chamber.
More specifically most of the enzymes
are
catalase and
peroxidase (Beheshti and McIntosh 2007).
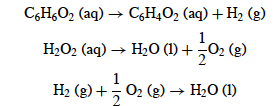 Upon
mixture of these enzymes with reactants from the reservoir
chamber, there is a production of oxygen gas and water from the
peroxides (Schwarcz 2010). Benzoquinone is also formed from the reaction between the
oxygen gas and hydroquinone (Schwarcz 2010). Not only is there a
production of irritating compounds, but the
overall reaction occurring in the reaction chamber is
ridiculously
exothermic as seen by the fact it has a change in
enthalpy of -794.2kJ/kg solution (Beheshti and McIntosh 2007).
This is exactly how the spray is able to heat up to 100°C in most
bombardier beetles (Beheshti and McIntosh 2007). In Metrius
contractus there is a notably lower temperature of spray
that
is generally closer to 55°C (Eisner et al. 2000). This can be
attributed to an evolutionary difference in how its jets
function (Eisner et al. 1977). Finally, after releasing the
advanced chemical mixture, the
channel leading outside of the body is closed, and the reservoir
is refilled with peroxides and quinone preparing the beetle for
the next attack (James et al. 2012).
Upon
mixture of these enzymes with reactants from the reservoir
chamber, there is a production of oxygen gas and water from the
peroxides (Schwarcz 2010). Benzoquinone is also formed from the reaction between the
oxygen gas and hydroquinone (Schwarcz 2010). Not only is there a
production of irritating compounds, but the
overall reaction occurring in the reaction chamber is
ridiculously
exothermic as seen by the fact it has a change in
enthalpy of -794.2kJ/kg solution (Beheshti and McIntosh 2007).
This is exactly how the spray is able to heat up to 100°C in most
bombardier beetles (Beheshti and McIntosh 2007). In Metrius
contractus there is a notably lower temperature of spray
that
is generally closer to 55°C (Eisner et al. 2000). This can be
attributed to an evolutionary difference in how its jets
function (Eisner et al. 1977). Finally, after releasing the
advanced chemical mixture, the
channel leading outside of the body is closed, and the reservoir
is refilled with peroxides and quinone preparing the beetle for
the next attack (James et al. 2012).
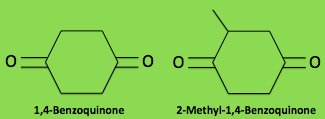 Aside from various similarities that relate Metrius contractus
to other organisms which are discussed in detail on the
Classification page, it also has some very thrilling unique
characteristics relating to its chemistry. Eisner et al. (2000)
discusses the numerous anomalies in the chemical make up of its
spray. The first strange discovery in this study was the
presence of
2-chlorobenzoquinone. This compound was the second
most concentrated in the spray which is unbelievable considering
that compound is hardly ever found in nature (Eisner et al.
2000). It is an
incredibly difficult compound to form. In the same study, it was
found that the primary quinone produced by Metrius contractus is
1,4-benzoquinone. This is different from every other bombardier
beetle as normally 2-methyl-1,4-benzoquinone is the primary
quinone. While they sound very similar, these are quite
different chemicals and make a huge difference in the overall
spray properties (Eisner et al. 2000).
Aside from various similarities that relate Metrius contractus
to other organisms which are discussed in detail on the
Classification page, it also has some very thrilling unique
characteristics relating to its chemistry. Eisner et al. (2000)
discusses the numerous anomalies in the chemical make up of its
spray. The first strange discovery in this study was the
presence of
2-chlorobenzoquinone. This compound was the second
most concentrated in the spray which is unbelievable considering
that compound is hardly ever found in nature (Eisner et al.
2000). It is an
incredibly difficult compound to form. In the same study, it was
found that the primary quinone produced by Metrius contractus is
1,4-benzoquinone. This is different from every other bombardier
beetle as normally 2-methyl-1,4-benzoquinone is the primary
quinone. While they sound very similar, these are quite
different chemicals and make a huge difference in the overall
spray properties (Eisner et al. 2000). It is for this reason that the spray of
Metrius contractus is so different from other beetles and tends
to hang around in the air making a cloud of toxic spray (Eisner
et al. 2000). Lastly in this study, it was found that the spray
of Metrius contractus also contains
conjugated dienes which are
not only also highly unlikely to find in nature, but would never
be expected to be in the same space as 1,4-benzoquinone without
reacting. A special mechanism using
stereochemistry to prevent
these compounds from completely reacting with each other has
been developed over time by these beetles (Eisner et al. 2000).
It is for this reason that the spray of
Metrius contractus is so different from other beetles and tends
to hang around in the air making a cloud of toxic spray (Eisner
et al. 2000). Lastly in this study, it was found that the spray
of Metrius contractus also contains
conjugated dienes which are
not only also highly unlikely to find in nature, but would never
be expected to be in the same space as 1,4-benzoquinone without
reacting. A special mechanism using
stereochemistry to prevent
these compounds from completely reacting with each other has
been developed over time by these beetles (Eisner et al. 2000).
Metrius contractus is
the chemical mastermind of the natural world, and that has some
astonishing
applications. Feeling flabbergasted by these organic
chemist braniac beetles? Be sure to refer to the
Explanation of Terms page. If you want to know more about
the chemical quinones found in bombardier beetles, take a look
at what the
U.S.
Environmental Protection Agency has to say about them.
Revisit Reproduction or check out some Applications of all this information.
