Prions
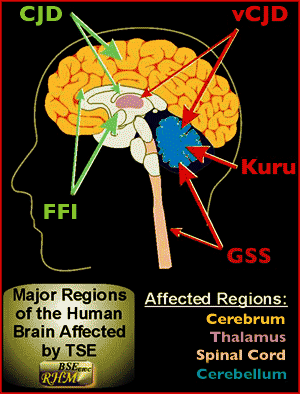
Pathogenesis of Prion Diseases
*CJD is the specific example here, but the pathogenesis is very similar among all TSEs.
*This pathogenesis process was taken from Northeastern Ohio's Universities College of Medicine Neuropathology.
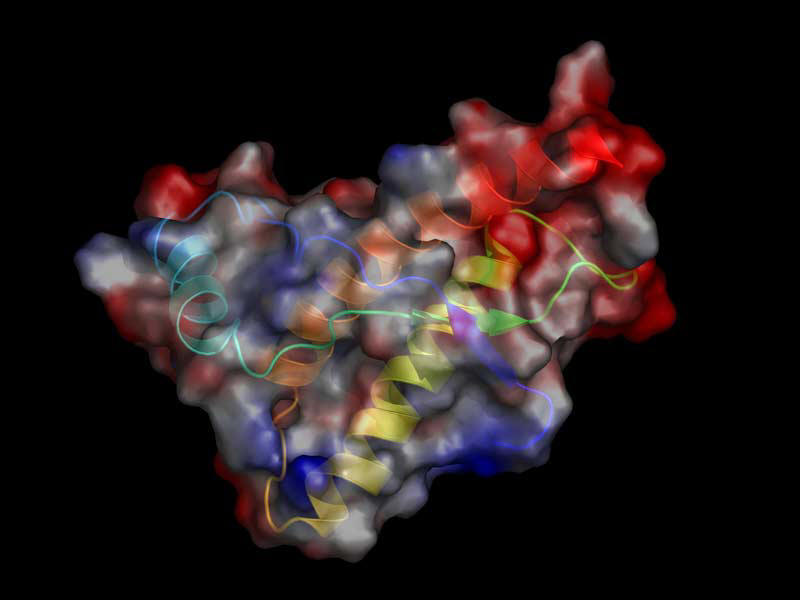
- Misfolding
of the normal prion protein (PrPC) converts it to
an insoluble, protease resistant isoform (PrPSc),
which precipitates as amyloid.
loss by some unknown mechanism.
- In
familial CJD, mutations of the Prion protein gene cause
prions to misfold.
It is not clear what causes sporadic CJD. Polymorphisms of the prion protein gene at codon 129 increase susceptibility and influence the phenotype of sporadic CJD.
- In iatrogenic and variant CJD, PrPSc introduced into the brain induce PrPC to misfold. Endogenous PrPSc produced in familial and sporadic CJD also has the same effect.
Among humans the different TSEs include Creutzfeldt-Jakob disease, Gerstmann-Straussler syndrome, Fatal familial insomnia, and Kuru. In other mammals there are well known prion diseases such as Mad Cow Disease, Scrapie, Chronic wasting disease, and others. However, as research among different organisms continues, it is becoming clear that many of them suffer from prion presence and TSEs.
There is
no known treatment for TSEs, prevention is the key! Take a
look at this short
slideshow which shows an animation
indicating how the proteins are changed in shape and function.