The structure of Mycoplasma pneumonia is very unique and infinitely interesting. Besides the fact M. pneumonia is one of the smallest free living organisms known, they also lack a cell wall and still maintain cell rigidity.
The key to M. pneumoniae’s
ability to maintain regular cell morphology is the presence of a fibrous
cytoskeleton deep to the cell membrane. The cytoskeleton is made up
many different fibrous proteins. Each “fiber” is 80-100 wide by
250-300nm long. The proteins that make up the cytoskeleton are diverse
in function. Some of these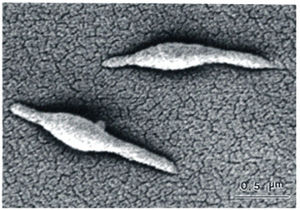 functions include: support, motility, translation, heat shock response,
energy metabolism, and attachment to host cells. In addition to these
proteins, a few lipoproteins can also be found in the cytoskeleton. The
protein thought to be responsible for motility in M. pneumoniae
is the “Neimark protein”. The Neimark protein is much like the actin
molecule found in the muscle of many animals and is thought to function
much the same. Other important proteins found in the cytoskeleton
include the so called “adhesion proteins”. These proteins are
responsible for the binding of the M. pneumoniae cell to the host
cells. The adhesion proteins include the P30 protein, P40 protein, and
P90 protein. Without the presence of any of these proteins, the M.
pneumoniae cells cannot bind to host cells. These proteins
work in conjunction with the "attachment organelle" to bind to host
cells. The attachment organelle is also thought to play a major
role in reproduction. M. pneumoniae reproduces via binary fission.
functions include: support, motility, translation, heat shock response,
energy metabolism, and attachment to host cells. In addition to these
proteins, a few lipoproteins can also be found in the cytoskeleton. The
protein thought to be responsible for motility in M. pneumoniae
is the “Neimark protein”. The Neimark protein is much like the actin
molecule found in the muscle of many animals and is thought to function
much the same. Other important proteins found in the cytoskeleton
include the so called “adhesion proteins”. These proteins are
responsible for the binding of the M. pneumoniae cell to the host
cells. The adhesion proteins include the P30 protein, P40 protein, and
P90 protein. Without the presence of any of these proteins, the M.
pneumoniae cells cannot bind to host cells. These proteins
work in conjunction with the "attachment organelle" to bind to host
cells. The attachment organelle is also thought to play a major
role in reproduction. M. pneumoniae reproduces via binary fission.
Surrounding the cytoskeleton is the plasma membrane. Unlike the lipid bi-layer of most organisms, M. pneumoniae has a tri-layered cell membrane that contains serol-like molecules that function to add support.