|
|
 |
 |
|
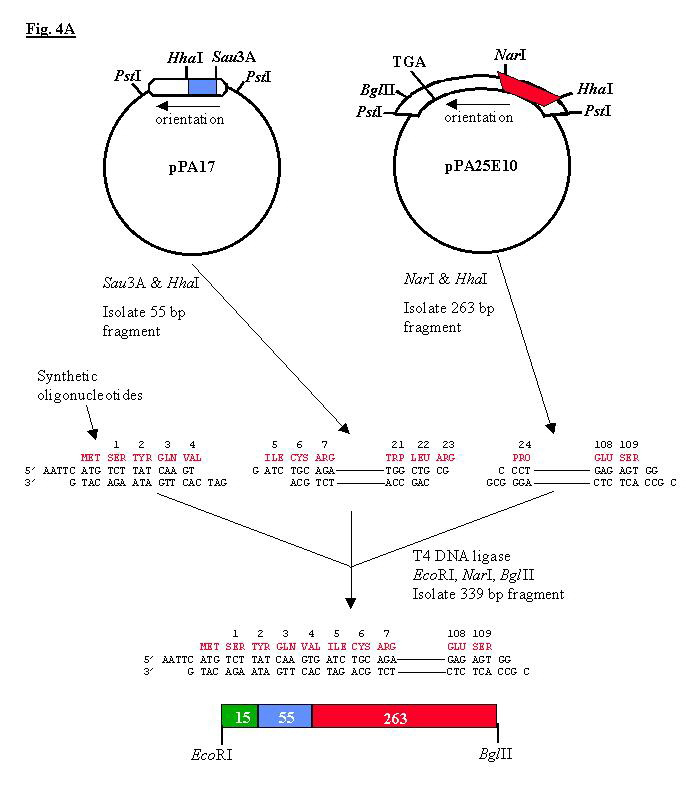
Fig. 4A Construction of a plasmid coding for the direct expression of mature t-PA in E. coli. 50 ug of plasmid pPA17 was digested with Sau3A, HincII and HhaI and electrophoresed on a 6% polyacrylamide gel; ~0.5 ug of the 55 bp Sau3A-HhaI fragment was recovered. Similarly, ~3 ug of the 263 bp HhaI-NarI fragment was purified from 80 ug of clone pPA25E10. All digests were performed at 37oC for 1 h and reaction products resolved and electroeluted from 6% polyacrylamide gels. The two indicated deoxynucleotides 5´ dAATTCATGTCTTATCAAGT (I) and 5´ dGATCACTTGATAAGACATC (II) were syntesized on a solid phase synthesizer. 100 pmol of oligonucleotide II was phosphorylated with T4 polynucleotide kinase. After phenol and chloroform extraction, the phosphorylated oligomer II and the 5´-hydroxyl oligomer I were combined with 0.5 ug of the eluted 55 bp Sau3A-HhaI fragment and 2 ug of the 263 bp HhaI-NarI fragment. These fragments were ligated at room temperature for 4h in buffer containing 0.5 mM ATP and 1,000 U of T4 DNA ligase. The mixture was then digested for 1 h with 48 U NarI, 20 U EcoRI, and 40 U BglII (to eliminate polymerization through ligation of cohesive Sau3A termini). The 338 bp fragment was isolated by electrophoresis on a 6% acrylamide gel and electroelution. |
 |
|
|
 |
 |
 |
|
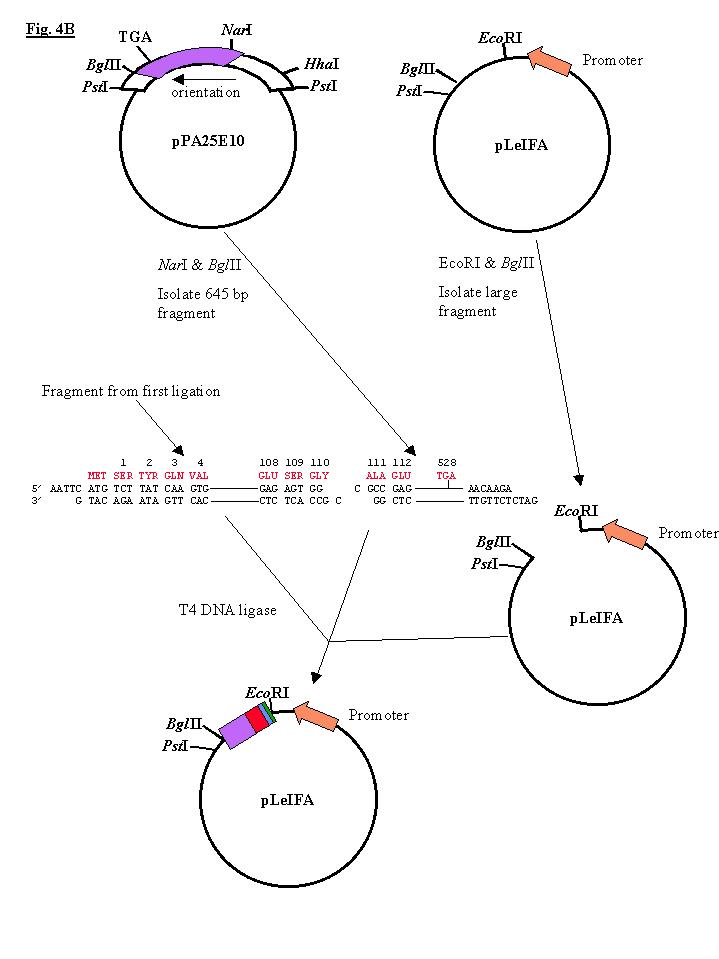
Fig. 4B The remainder of the t-PA coding sequences (aa 111-528) were isolated on a 1,645 bp fragment by digesting plasmid pPA25E10 with NarI and BglII. The plasmid pLeIFA was digested with EcoRI and BglII, producing a large 4,200 bp vector fragment. Both fragments were separated and purified as described above. For the final construction, 80 ng of EcoRI/BglII pLeIFA was ligated with 100 ng of the 1,645 bp NarI/BglII fragment and 20 ng of the 338 bp EcoRI/NarI fragment at room temperature for 1o h. This ligation mixture was used to transform E. coli K-12 strain 294. Plasmid DNA was prepared from 38 transformants and digested with EcoRI, 10 of these plasmids contained the desired 600 and 472 bp EcoRI fragments. DNA sequence analysis verified that one of these plasmids had the desired nucleotide sequence at the junctions between the trp promoter, synthetic DNA and cDNA. |
|