|
Current Level |
||||||||||
|
|
||||||||||
|
Previous Level |
||||||||||
|
|
||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Construction and idenfication of bacterial clones containing t-PA cDNA sequences. RNA from gel slice 7 (Fig. 2A) was used to prepare double-stranded cDNA. Ten ug of poly(A)+ mRNA were mixed with a solution containing all four dNTPs (500 uM each), 10 mM DTT, 50 ug/ml oligo(dT)12-18, and 200 U of AMV reverse transcriptase. The reaction was incubated at 42oC for 1.5 hr. Proteins were removed by phenol/chloroform extraction and the DNA precipitated with ethanol. The single stranded cDNA was converted into double-stranded cDNA by resuspending the pellet in buffer containing 10 U/ml RNaseH, 250 U/ml DNA polymerase I, and 50 U/ml DNA ligase and incubating the reaction at 14oC for 12-16 hours. The cDNA was fractioneated by size, and material longer than 350 bp was ligated with adapters containing a PstI overhang using T4 DNA ligase. The resulting cDNAs were ligated with PstI treated pBR322 using T4 DNA ligase at 14oC for 12 hours. The resulting constructs were transformed into calcium competent E. coli K-12 strain 294 by heat shock for 90 seconds at 42oC, approximately 4,600 transformants were observed. back to questions To prepare a specific DNA hybridization probe, we determined the amino acid sequences of several tryptic fragments of t-PA purified from melanoma cells. This information permitted the design of synthetic deoxyneucleotides potentially complementary to a specific region of t-PA mRNA. The amino acid seqeunce (Trp-Glu-Tyr-Cys-Asp) was selected for preparation of a probe because it required the synthesis of only eight tetradecanucleotides (dTCA/GCAA/GTAC/TTCCCA) to account for all possible anti-coding seqeunces. This pool of 14-mers was labelled with 32P and used to screen the 4,600 cDNA clones by in situ colony hybridization. Plasmid DNA was isolated from 12 colonies that gave a positive hybridization signal. DNA sequencing was performed on the cDNA inserts from each of these clones by the dideoxy chain termination procedure. Only one cDNA insert, that of colony 25E10, contained sequences which could code for the amino acid sequence of the tryptic peptides of melanoma t-PA. The entire cDNA in the plasmid (pPA25E10) was sequenced and found to be 2,304 bp long. The sequence contains an open reading frame encoding a protein of 508 amino acids and a 745 bp 3´ untranslated region. back to questions
Preparation of a colony library containing NH2-terminal t-PA sequences. The cDNA clone pPA25E10 was not a full-length copy of t-PA mRNA as it lacked the t-PA NH2-terminal coding sequences determined by amino acid sequencing of the purified protein. Therefore, it was necessary to produce cDNA clones containing the 5´ portion of the t-PA mRNA. A 16 bp long deoxyoligonucleotide complementary to nucleotides 256-271 of the t-PA mRNA was synthesized (dTTCTGAGCACAGGGCG). This hexadecanucleotide was used to prime cDNA synthesis of fraction 7 mRNA and 1,500 cDNA clones were obtained. To determine which of these cDNA clones contained a complete NH2-terminal coding sequence a probe spanning the 5´ end of pPA25E10 was needed. We therefore isolated a genomic clone for t-PA from a human gene library and used this as a hybridization probe to identify primer extended cDNA clones containing NH2-terminal coding sequences. The first step in this process was to determine wheter only a single homologous t-PA gene was present in human genomic DNA. Southern hybridizations were performed using high molecular weight DNA which had been digested with various restriction endonucleases. The 32P-labelled cDNA probe used was a 232 bp RsaI-PstI fragment (nucleotides 338-620) prepared from the 5´ end of the cDNA insert of clone pPA25E10. Two endonuclease digestion patterns provided only a single hybridizing DNA fragment BglII (5.7 kilobase pairs, kbp) and PvuII (4.2 kbp). Two hybridizing DNA fragments were observed with HincII (5.1 and 4.3 kbp) (Figure 3C). Comparison of these data with the cDNA restriction map (Fig. 3A) suggests that there is only one t-PA gene in the human genome and that this gene contains at least one intervening sequence. Approximately 106 plaques of a l human genomic library were screened with the 32P-labelled 232-bp RsaI-PstI fragment, 19 individual clones were isolated and the phage DNA was prepared. A 4.2 kbp PvuII fragment containing t-PA sequences was isolated from one of these clones, labelled with 32P and used to screen the 1,500 clones of the 5´ primer extended cDNA library. Plasmid DNAs were prepared from the 18 colonies which gave positive hybridization signals and these DNAs were bound to a nitrocellulose filter. To identify clones having cDNA inserts which overlapped with the cDNA insert of pPA25E10, the filter was hybridized with the 32P-labelled synthetic oligonucleotide (16-mer) used for teh original priming reaction. Of the 18 selected cDNA clones, 7 hybridized with the 32P-labelled 16-mer, however, on sequence analysis of the cDNA clones, only pPA17 was found to contain the complete t-PA NH2-terminal coding sequence and to overlap with the original clone 25E10. The cDNA insert of pPA17 is 271 bp long. It contains the 16-mer used to prime its synthesis, which permitted alignment with 25E10. back to questions |
|
|
|
|
|
|
|
The complete 2,530 bp cDNA sequence contains a single open reading frame, beginning with the ATG codon at nucleotides 85-87. This ATG is followed, 562 codons later, by a TGA termination triplet at nucleotides 1,771-1,773. The serine designated as aa 1 is based on NH2-terminal sequencing of purified melanoma cell t-PA. This serine is preceded by 35 aa, the NH2-terminal 20-23 of which probably constitute a hydrophobic signal peptide involved in secretion of t-PA. The remaining 12-15 hydrophobic aa immediately preceding the start of mature t-PA may constitute a `pro´ sequence, similar to that found in serum albumin. the 3´ untranslated region of 759 nucleotides contains the hexanucleotide AATAAA which precedes the site of polyadenylation in many eukaryotic mRNAs. The native t-PA molecule has 35 cysteine residues, and thus has the potential to be stabilized by 17 disulphide bridges. There are four potential N-glycosylation sites, located at Asn 117, Asn 184, Asn 218 and Asn 448 in t-PA. back to questions The procedure to express the full length cDNA insert of t-PA in E. coli is outlined in Fig. 4A. The common HhaI restriction endonuclease site shared by the cDNA inserts of both partial clones pPA17 and pPA25E10 permitted the reconstruction of the entire mature t-PA coding sequence. A 55 bp Sau3A-HhaI restriction fragment corresponding to aa 5-23 was isloated from the plasmid pPA17. A 263 bp NarI-HhaI fragment (aa 14-110) was isolated from the plasmid pPA25E10. Two synthetic oligonucleotides were designed to restore the codons for aa 1-4, incorporate an ATG translation initiation codon and create an EcoRI cohesive terminus. These fragments were then ligated together to form a 338 bp fragment coding for aa 1-110 of t-PA. This fragment and a 1,646 bp Sau3A-HhaI fragment from pPA25E10 were then ligated between the and sites of the plasmid pLEIFA to give the expression plasmid pt-PA (Fig. 4B). The cloned t-PA cDNA is transcribed under the control of a 300 bp fragment of the E. coli trp operon which contains the trp promoter, operator and the Shine-Delgarno sequence, but lacks the leader peptide ATG initiation codon. The plasmid was introduced into E. coli and recombinant human t-PA was expressed and purified. The t-PA activity (3-5 U/L) recovered from the E. coli extracts corresponds to 50-80 ug/L of culture. The published activity of purified human t-PA is 60,000 U/mg. As only functional activity was measured, it is possible that considerably more t-PA is present in these cells. In addition, because polypeptides synthesized in E. coli are not glycosylated, the t-PA synthesized by E. coli might have a specific activity different from that of authentic t-PA. back to questions |
|
Expression of active human tissue-type plasminogen activator in Escherichia coli. Qiu J, Swartz JR, Georgiou G. Appl Environ Microbiol 1998 Dec;64(12):4891-6 |
|
Occasionally, proteins that are not expressed in an active form in bacteria can be expressed in the simple eukaryote, yeast. Expression of high levels of human tissue plasminogen activator in yeast under the control of an inducible GAL promoter. Martegani E, Forlani N, Mauri I, Porro D, Schleuning WD, Alberghina L. Appl Microbiol Biotechnol 1992 Aug;37(5):604-8 High levels of tPA as inactive denatured protein have been expressed in bacteria, but the recovery of biological activity requires long and inefficient renaturation prodcedures. Attempts to express human t-PA in yeast usually yield a very low level of expression of a hyperglycosylated protein. In recent years our laboratory has been engaged in studying the expression of heterologous genes in budding yeast under the strong inducible hybrid promoter UASgal/CYC1. Under optimal growth conditions fairly high levels of expression (5-15% of total yeast protein) has been obtained with secreted proteins. In the present work we describe the expression of high levels of active h-tPA in yeast using the UASgal/CYC1 promoter. To obtain high expression we made fusions between the tPA cDNA and the leader sequence of the killer toxin of K. lactis present in the yeast vector YEp-sec1.
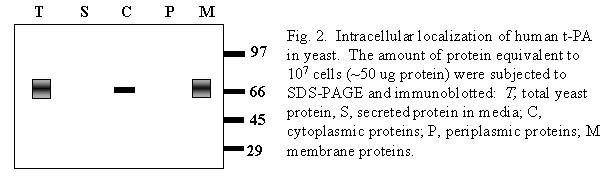
Methods Cells were grown in complete media with an initial carbon source of glucose. To induce expression of t-PA, a mixture of glucose/galactose (2:1) was used. Growth was determined by counting cells or as dry weight of biomass. A 1.97 kb BglII-BglII fragment of human t-PA cDNA obtained from the pPA11-4B plasmid (Fisher et al. 1985), corresponding to the sequence of the mature polypeptide, was ligated in the BamHI site of the yeast expression vector YEp-sec1. In the resulting plasmid (YptPAind1) the t-PA sequence was in frame with the leader peptide of the killer toxin of K. lactis, while the transcriptional termination and polyadenlyation sites were provided by the yeast 2u FLP gene. Results A simple fractionation of cellular components shows that the recombinant human t-PA was present almost exclusively in the crude membrane fraction (Fig. 2). A total activity of 50,000,000 U/L was obtained. Considering a specific activity of pure human t-PA of 500,000 U/mg, about 100 mg of active protein was produced per liter. Solubilization of t-PA was obtained by washing crude membranes with buffers of high ionic strength in the presence of 0.1% Tween 80, followed by ZnCl2 precipitation and dialysis. The best specific activity was 160,000 U/mg. back to questions |
 |
|
|
|
