Interactions
Coprinopsis atramentaria is detritivorous, meaning it lives off of dead organic material. This provides great ecological benefits, like reducing the amount of decaying organic material, and recycling the nutrients trapped in dead debris, eventually reintroducing the nutrients back to the food-web.
 Edible: Being saprobic (detritivorous), means it is not parasitic
and does not form any mutualistic or commensal symbiotic
relationships with other organisms. However,
they do interact with other organisms, for example
they compete with other fungi and plants for the
nutrients and water in the soil. They also
commonly serve as a habitat for many organisms like
bacteria and protists. Many mushrooms
even serve
as a food source for other organisms like insects, bacteria, smaller mammals, like pigs,
squirrels, chipmunks, and last but not least, humans. They are
actually very nutritious and contain copper, iron,
vitamin c, and more potassium per gram than bananas!
They are also a low calorie/low cholesterol food
that contains a significant amount of fiber.
Edible: Being saprobic (detritivorous), means it is not parasitic
and does not form any mutualistic or commensal symbiotic
relationships with other organisms. However,
they do interact with other organisms, for example
they compete with other fungi and plants for the
nutrients and water in the soil. They also
commonly serve as a habitat for many organisms like
bacteria and protists. Many mushrooms
even serve
as a food source for other organisms like insects, bacteria, smaller mammals, like pigs,
squirrels, chipmunks, and last but not least, humans. They are
actually very nutritious and contain copper, iron,
vitamin c, and more potassium per gram than bananas!
They are also a low calorie/low cholesterol food
that contains a significant amount of fiber.
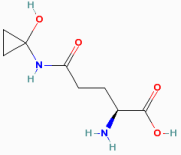 Toxicity: But there is a catch, Coprinopsis atramentaria is
known for being poisonous when consumed with
alcohol! This mushroom isn’t a directly toxic
organism, it actually metabolizes the chemical coprine (illustrated to the left) into a chemical
that interferes with the breakdown of ethanol,
causes a build up of aldehydes in your bloodstream,
thus making the alcohol poisonous. The effects of
this are similar to that of the drug disulfiram (antabuse).
This drug is used to treat people with an alcohol
dependence because it causes symptoms like severe
nausea, vomiting, and headaches upon alcohol
consumption. Similar to this drug, after drinking
alcohol before, with, or after the mushroom
Coprinosis atramentaria, the onset of symptoms will
occur anytime from 5 minutes, to two hours after
consumption, and last anywhere from 45 minutes to a
couple of hours. These symptoms include an elevated
heart rate, flushing of the skin on the upper half
of the body, headache, rapid breathing, weakness,
dizziness, nausea and vomiting. Keep in mind that
the coprine can keep your body susceptible to this
poisonous interaction for up to a few days, and in
rare cases, up to a week.
Toxicity: But there is a catch, Coprinopsis atramentaria is
known for being poisonous when consumed with
alcohol! This mushroom isn’t a directly toxic
organism, it actually metabolizes the chemical coprine (illustrated to the left) into a chemical
that interferes with the breakdown of ethanol,
causes a build up of aldehydes in your bloodstream,
thus making the alcohol poisonous. The effects of
this are similar to that of the drug disulfiram (antabuse).
This drug is used to treat people with an alcohol
dependence because it causes symptoms like severe
nausea, vomiting, and headaches upon alcohol
consumption. Similar to this drug, after drinking
alcohol before, with, or after the mushroom
Coprinosis atramentaria, the onset of symptoms will
occur anytime from 5 minutes, to two hours after
consumption, and last anywhere from 45 minutes to a
couple of hours. These symptoms include an elevated
heart rate, flushing of the skin on the upper half
of the body, headache, rapid breathing, weakness,
dizziness, nausea and vomiting. Keep in mind that
the coprine can keep your body susceptible to this
poisonous interaction for up to a few days, and in
rare cases, up to a week.
The True Culprit:
The novelty of
the chemical coprine, is in the fact that it is the first
example of an enzyme inhibitor that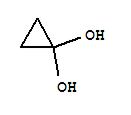 contains a cyclopropyl ring at the
oxidation state of cycloproanone. So first off, the
side effects of this sensitivity to alcohol are
actually caused by the inhibition of aldehyde
dehydrogenase, which a vital enzyme responsible for
the metabolism of ethanol. In essence, coprine
isn’t toxic, however it does modify and amplify
alcohol’s toxicity. Coprine produces a metabolic
product of cyclopropanone hemiaminal, that
ultimately becomes the true culprit, cyclopropanone
hydrate (illustrated to the right). This acts as a
substrate analogue and is indeed ultimately the
chemical responsible for the inhibition of aldehyde
dehydrogenase. This is the interaction that causes
contains a cyclopropyl ring at the
oxidation state of cycloproanone. So first off, the
side effects of this sensitivity to alcohol are
actually caused by the inhibition of aldehyde
dehydrogenase, which a vital enzyme responsible for
the metabolism of ethanol. In essence, coprine
isn’t toxic, however it does modify and amplify
alcohol’s toxicity. Coprine produces a metabolic
product of cyclopropanone hemiaminal, that
ultimately becomes the true culprit, cyclopropanone
hydrate (illustrated to the right). This acts as a
substrate analogue and is indeed ultimately the
chemical responsible for the inhibition of aldehyde
dehydrogenase. This is the interaction that causes
 aldehyde poisoning.
aldehyde poisoning.
Coprine for Dummies: So essentially, the break down of coprine in your body results in a chemical that is similar in structure to a different chemical that is vital in the breakdown of ethanol, which ultimately distracts the enzymes that break down alcohol by causing a diversion. This leads to aldehyde poisoning (build up of alhedydes in your blood).
The Dirty Details: Here are some dirty details of the chemical interactions. An enzyme catalyzes the dehydration of cyclopropanone hydrate into cycloproanone. Cycloproanone binds to the active-site thiol on the enzyme aldehyde dehydrogenase, and creates a kinetically stable thiohemiketal. Thiohemiketals are analogous (similar to) thiohemiacetals, which are obligatory intermediates in the enzyme-catalyzed oxidation of aldehydes. Thus Cyclopropanone hydrate ultimately lessens the amount of enzymes available to act on thio reagents, which causes a build up of aldehyde levels in the blood and impedes the metabolism of ethanol. This is what causes the "poisionous" reaction that results in aldehyde poisoning.
This has all been very interesting, but you're in luck, because there's more! Click here to learn what "going inky" really means.
