|








| |
|
Mycobacterium
tuberculosis |
|
Adaptations |
Mycobacterium tuberculosis
is a highly successful pathogen because of a number of structural and
physiological adaptations. First of all, the bacterium possesses a
thick, waxy cell wall that is rich in lipids, many of which are mycolic
acids (Figure 1). A similar type of cell wall is observed in the
saprophytic bacteria, which are close relatives of M. tuberculosis.
Since saprophytic bacteria feed on dead or decaying organic matter and
live in a soil environment, a lipid-rich cell wall allows the organisms
to resist desiccation. Although M. tuberculosis does not live in
the soil but rather within the macrophages of a human or animal host, a
high lipid content proves equally important in the survival of the
bacterium. When macrophages engulf M. tuberculosis bacilli that
are inhaled into the lungs, the macrophages are unable to destroy the
bacteria because their lipid-rich cell walls serve as protection.
|
|
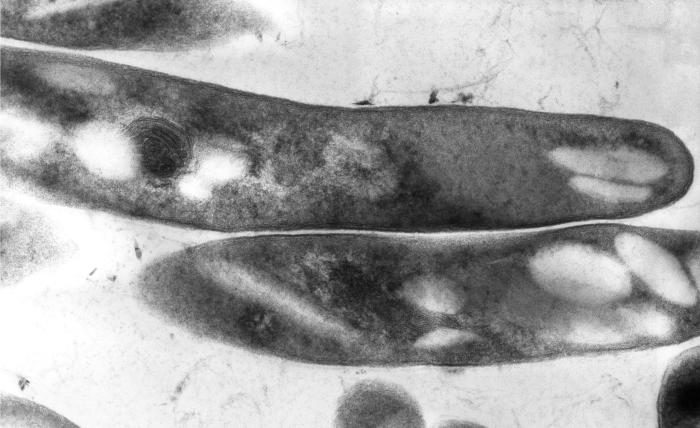 |
Figure 1. Transmission electron micrograph of
Mycobacterium tuberculosis bacilli. The lipid-rich cell wall
of M. tuberculosis is a trait shared with the saprophytic
bacteria.
|
|
Once inside the macrophages,
M. tuberculosis has the ability to alter the immune response that
would normally take place. After phagocytosis, a macrophage invaginates
its cell membrane around the foreign particle that was just engulfed to
form a vacuole called a phagosome. In phagosome maturation, lysosomes
(organelles that contain enzymes for intracellular digestion) fuse with
the phagosome and release their hydrolytic enzymes. (“Hydrolytic” stems
from the word “hydrolysis,” which refers to chemical reactions that
split compounds with water.) By becoming acidic and hydrolytically
active, the phagosome is then able to digest the foreign particle.
Through mechanisms that are still being questioned, Mycobacterium
tuberculosis can stop phagosome maturation from progressing and thus
maintain the macrophage as a suitable environment in which to live.
|
Figure 2. Photograph of four antibiotic drugs used to treat
tuberculosis. From left to right:
isoniazid, rifampin, pyrazinamide, and ethambutol. |
The adaptive ability of
Mycobacterium tuberculosis is also evident in cases of antibiotic
drug resistance. If a person skin-tests positive for tuberculosis but
does not show any signs of disease, he or she is treated for six to nine
months with a drug called isoniazid. (See the “Diagnosis” section for
more information on tuberculin skin tests.) Cases of active
tuberculosis disease require six to nine months on three to four
antibiotics, the most popular being isoniazid, ethambutal, rifampin, and
pyrazinamide (Figure 2, above). Multiple antibiotics are needed because isoniazid, a mycolic acid inhibitor, kills the rapidly growing bacteria
while the other three drugs target slower-growing and dormant bacteria.
When individuals fail to comply with antibiotic therapy, either by not
taking their medications regularly or by not taking all of the required
doses, antibiotic resistance comes into play. In such cases, M.
tuberculosis bacilli are exposed to a concentration of antibiotics
that is just below the level needed to effectively kill the bacteria.
Therefore, the bacteria build up tolerance, making them less sensitive
to the effects of the drugs (Figure 3).
|
|
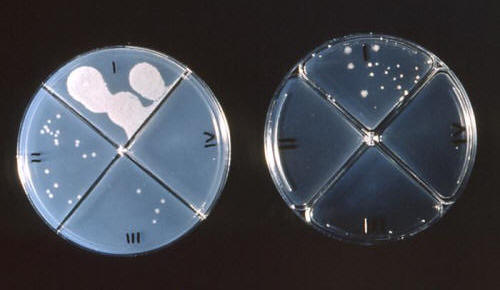 |
Figure 3. Mycobacterium tuberculosis colonies
growing on agar which has been treated with various antibiotic drugs.
Field 1 of the plate on the left represents the control group.
|
|
Preventing antibiotics from being activated in the first place is
another way in which Mycobacterium tuberculosis can become drug
resistant. The enzyme catalase, which the body naturally produces to
convert hydrogen peroxide to water and oxygen, also acts on isoniazid,
essentially “turning on” the drug. Activated isoniazid works by
inhibiting mycolic acid synthesis and, thus, breaking down the cell wall
of M. tuberculosis. If the mycobacteria stop the production of
catalase, however, isoniazid never gets activated after entering the
body. |
|