Toxin
What makes this mushroom so deadly?
The toxin that resides in Cortinarius s. is so deadly it has earned it a spot in the most lethal toxins in nature. So far studies have not been able to isolate the exact chemical that gives this mushroom its lethal bite. But in more recent studies have identified it to be a Nephrotoxin, and has a nearly identically structure to Orrelanine, another deadly chemical in certain toxins. For the purpose of quatlative comparison study, looked at Canaries orellanus another closely related species. One study had mice that were administered a purified form of the Orrelanine toxin by mouth. Two toxins were identified: one main fluorescent toxin and one non-essential non fluorescent toxin. Both of these toxin were identified by mass spectrometric, nuclear and magnetic resonance analysis. The mice were given 9 mg/g of the main toxin and the same amount of the second toxin. Results showed that the main fluorescent toxin was the was vital in mice that showed poisoning. The second non-Fluorescent toxin proved to be ineffective.
What is the chemical structure?
Orrelanine has a bi-byriyl structure with positively charged
Nitrogen atoms(known herbicide) Quaternary Nitrogen atoms initiate a
redox reaction inside the host. The removal of electrons form Oxygen
generates superoxide's and peroxides. These are believed to cause the
cell necrosis in liver cells that we typically see in a Cortinarius
s. poisonings. The agreed upon lethal dose in average man in
100-200g. Typically Cortinarius o. has about 14mg of toxin per gram of
tissue.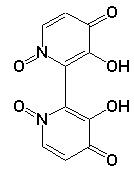
For more information about the exact pathology of Orrelanine visit pathology section under my Interactions page
Some Mushrooms Produce toxins that actually draw a mass appeal to use of the fungus. The hallucinate compound LSD was originally derived from Claviceps purpurea.