Venom
Difference
between Venom and Poison
Venom is a
toxin that is produced by a organism and is actively used to cause
harm through their toxin's chemical properties. Venoms are developed
for both predation and as a defense strategy and can be deployed in
many different ways such as fangs, stingers, cnidocytes, and many
more, all of which work by injection.
Find out what some of the most venomous animals in world are at
Environmental Graffiti!
Poison is also a toxin, but it cannot be actively used. Poisons are
typically used as a defense strategy for organisms to avoid
predation and are usually absorbed through tissues rather than being
injected. The toxins of poisonous animals can be absorbed through
epithelial linings such as in the gut or through the skin. Check out
Dendrobates azureus (Blue Poison Dart Frog), a
poisonous frog.
Find out what some of the most poisonous plants in the world are at
Green Buzzz!
Venom and Pheromone
Secretion Gland

The original use for the venom glands of army ants is thought to be
for the production of proteinaceous compounds that were used on the
eggs. The compounds were supposed to create an adhesive coating
around the eggs to bind them to a substrate. The Dufour gland, which
is responsible for the production of the venom, is attached to the
sting of the sterile workers. The Defour gland is found in all
Hymenoptera and is used for multiple purposes. Some specie members
of the Hymenoptera use the venom to subdue their prey, however there
are many others that are as, if not more, important secondary
functions. These include the production of defensive allomones,
other deterrents, and communication pheromones the most crucial
product without which the ants’ colony would fall apart.
The Venom
The actual chemical used by the ants in their venom is formic acid,
which accounts for up to 60% of the venom. The average amount of
formic acid produced by ants is approximately 600 ug, but can be up
to 2 mg per ant. Formicine ant’s venom does not contain any other
voltaic compounds other than formic acid.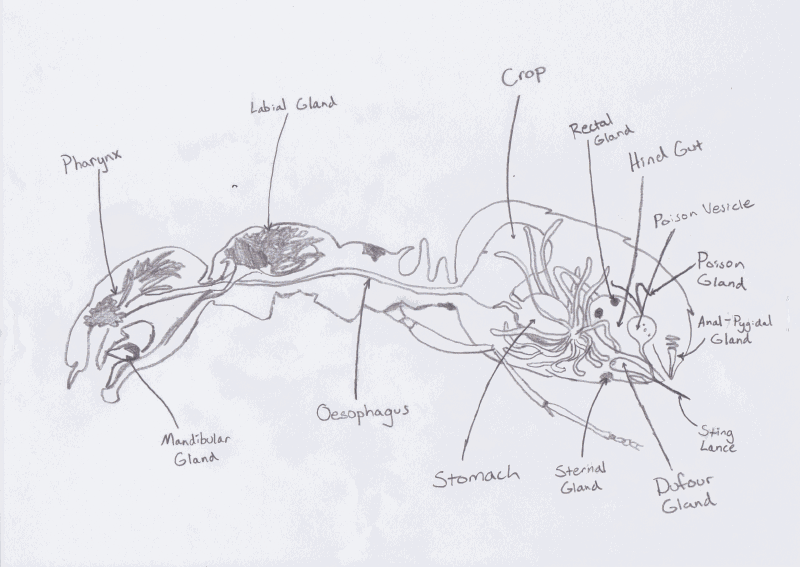
Check out the the venom in this interesting organism
Cyanea capillata
(Lion's Mane Jellyfish)
Venom Secretion
Formic Acid is synthesized in the Defour gland and starts by
converting serine to glycine (as shown below) by donating its
B-carbon to tetrahydrofolic acid. There after the methylene
tetrahydrofolate produced is oxidized to methylidyne form. Next, the
compound is hydrolyzed to 10-formyltetrahydrofolate and then formic
acid and another tetrahydrofolic acid to regenerate more formic
acid.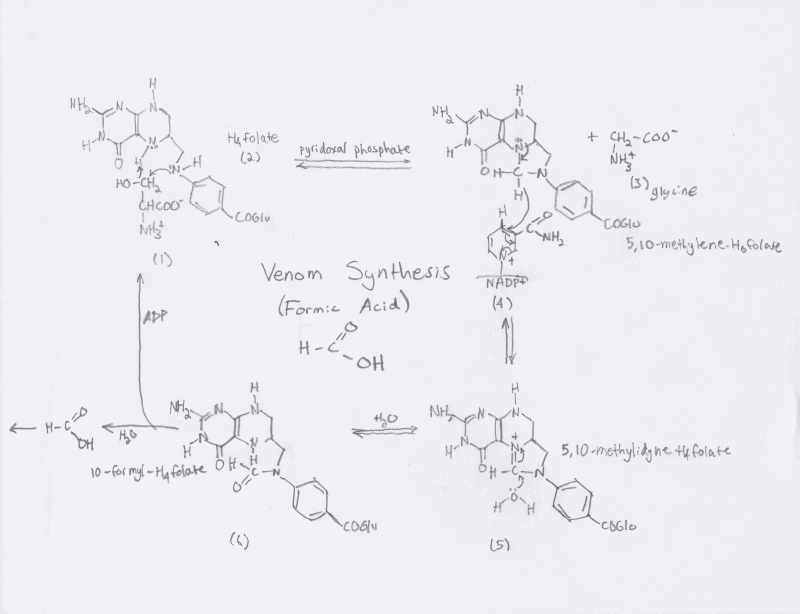
Are they dangerous?
The army ant sting is not particularly dangerous to humans unless
the individual that gets stung has an allergic reaction to the
venom, such as being stung by bees or wasps.